Notes collected from the Climber.Org Gear Forum, which generally reached the consensus that "it all depends on what you need":
Note from Roger in 2011: There is a new and very interesting rechargeable battery technology out there that is not talked about on this page. The technology I'm talking about is NiZn which puts out 1.6v and is available in AA and AAA formats. I'd be curious to know how they stack up against the Lithiums, Alkalines, and NiMH for cold weather performance. I'm also curious to know if they could be used in an avalanche beacon. I know that Barryvox offers new firmware which allows you to get accurate battery life readings for Li or Alkaline batteries but I don't know if either of those settings would work ok for the NiZn batteries. I have sent them an e-mail to find out if one can safely use NiZn batteries in their avalanche beacons or if they're looking into it at all.
Richard Schwaninger wrote:
I use the [Petzel] Zoom on longer trips and I always use the 4.5 V battery. It lasts...
well, I don't quite know [how long] since I replace the battery before each trip.
Paul Wilson wrote:
My old headlamp uses 2 AAA batteries. And I use fresh ones for trips that
need a full charge. The used batteries go on the shelf and I use them for my
mag light in my shop and the battery eating GPS. I seem to have a good supply
of used batteries.
Steve Eckert wrote:
That's the big problem with the big battery. I prefer smaller batteries
that I don't mind carrying spares for. The big ones are so heavy I've
almost never seen anyone carry a spare, but I have seen a couple of
DEAD big batteries because the headlamp turned on in a pack and ran
all day. Rhetorically, what do you do with all the 1/4-used battery packs?
I switched from expensive to cheap alkalines because I always carry
spares and I almost never run the first set down. The
cheap ones are lighter, so this way I'm carrying less weight.
That's not going to be reflected in ANY manufacturer spec!
Craig Smith wrote:
I always keep a set of regular lithiums in my pack as an emergency backup.
These will last for years w/o self-discharging, and are nice to use if
your rechargeable batteries run down before the end of the day.
Kevin Craig wrote:
I tried NiMH in my Garmin (eTrex's and 12XL).
There is some kind of incompatibility between the battery
meter algorithm in the Garmins and the 'power curve' or
whatever it's called for NiMH batteries that causes them
to always register as much less than 'full' even with fresh
batteries right out of the charger. Since I like to know
how much juice I really have left, I abandoned them in
favor of the LiIon which seem to work OK.
Craig Smith wrote:
Regarding batteries for the Garmin GPS: I have an eTrex and use rechargeable
Nickel metal hydride batteries most of the time. Works fine with very
reasonable battery life. As someone else mentioned, the output voltage of the
NiMH batteries is lower than the alkaline or lithium for which the eTrex is
designed, so the battery capacity bar graph will read less than 'full' even with
a newly charged NiMH battery set. But the life will be as good or better than
a set of alkalines.
Rich Feldman wrote:
An independent issue is the low-voltage cutoff of the equipment.
NiMH drop to 1.2 volts early in the discharge, but hit 1.1
just before exhaustion. With alkalines, most of the energy is
delivered above 1.25 volts, but you are throwing away a useful
fraction if you stop at 1.2 or even at 1.1.
Alan Ritter wrote:
I use the NiMH batteries exclusively in my digicam (Nikon 990). It has been
my experience with my Garmin GPS II+ that the NiMHs don't last as long in
the GPS as regular alkalines, which is just the opposite of the digicam. It
may have to do with the respective power supplies and what their minimum Vin
requirements are.
Richard Vassar wrote:
Battery capacity, especially for alkaline cells, is a function of the
current drain from the load. In high current drain applications, e.g.
flashlights, CD players, digital cameras, lithium AA cells have a
significantly higher mAh capacity than alkaline batteries.
Kevin Craig wrote:
Richard, thanks. That squares with my experience using lithiums
in FRS radios, headlamps, GPS etc. all high-drain applications.
Rich Feldman wrote:
Yeah, what Alan just said about NiMH outlasting alkalines
in a digicam but the opposite in radio receivers! A while back
I posted an explanation here, with URL's to battery technical
data (e.g. under duracell.com,
look for OEM information).
The Mallory charts give the AA size alkaline cell a capacity of
about 2800 mAH, but that's at room temperature, under light
loading, with end of life at about 1.0 or 0.9 volt.
Under similar conditions the NiMH are around 1500 mAH as Alan says.
But if you draw over 500 mA, like the Coolpix with monitor
on, the alkaline really sucks, and dies after less than 900 mAH.
This is where NiMH (and NiCd) excel: the ability to deliver most
of their energy at relatively high currents (even the 1 hour rate
is no sweat). (By the way, the coolpix is very tolerant of battery
voltage variations, in fact draws more current at lower voltage,
which implies a switching-mode DC-DC converter).
I don't know how lithium cells stack up, but in the previous
thread here, someone gave us a URL to tech data. At low current
they outlast alkalines, and much more so at low temperature.
Don't know about their fast-discharge performance, but many
digicams now come with a starter set of lithium cells. (often
each 3-volt cell is packaged to replace a side-by-side pair of AA's).
Paul Wilson wrote:
The Lithiums are much better for high current draw as in a
photoflash usage wherein the battery has a significant recovery
time between flashes and rewinds. Alkalines perform best when
subjected to low steady current draw, as in a gps or modern
LED headlamp. All the reports I can find about Lithiums having
longer life at normal temps are for usage in cameras or other
intermittent, high current applications. Can anybody provide
reports comparing low current draw between the two different
batteries? Real info would be better than the photo people
testimonials. If someone has Backpacker from some time last
year they compared Lithiums against alkaline's. Dont know about
current draw or temperature, but I was told it was a low temperature test.
BTW, I would not consider my LED headlamp as a high current drain
device with a alkaline battery life or about 20 hours. My gps has
similar low drain characteristics. Even my old halogen lamp was
good for 6-8 hours on good old low mAh alkalines.
Steve Eckert wrote:
Battery capacity depends STRONGLY on
temperature (which most people recognize) but ALSO on
current drain (which few are aware of).
Some alkaline batteries have a longer life in a clock
than sitting on the shelf not providing current at all.
The slow discharge keeps them from leaking.
Other batteries appear to discharge completely when used
in a bright (high-current) headlamp, but if you take them
out for an hour or two they will magically 'recharge' and
you can get more use out of them. Sort of like sopping
water off the floor only to find that more water seeps
out of the soaked walls. That's the kind of battery that
would work better in a low-current application.
Those ratings like 3000mAh, mean '3 amp-hours'. That
can TECHNICALLY be a 3 amp current provided for 1 hour, or
1 amp provided for 3 hours.... but good documentation
requires keeping those two numbers separate. They will NOT
be the same. You can't JUST look at the mAh rating. You've got
to know whether it was tested with high or low current,
intermittent or steady drain, and at what temperature.
Alan Ritter wrote:
Steve is quite correct. If you go to the battery manufacturers'
WWW sites (energizer.com
for instance) and look up the technical data on batteries,
you will find that alkaline batteries have vastly
different energy content depending upon the drain to
which they're subjected. By 'vastly', I mean 3:1 or
more, getting worse and worse as the drain goes up.
Alkalines are also more temperature sensitive than
lithium cells.
Part of this has to do with the internal impedance
(resistance) of the battery, part to do with the
chemistry. That's why alkalines are absolutely worthless in
high-drain applications like digital cameras but work
great in relatively low-drain applications like LED
headlamps, Walkman radios (not CD players), etc.
As usual, it's a matter of matching the tool to the
task. I have a couple of small single-LED flashlights
that run off 9V batteries. Using them fairly
regularly (but briefly) on Scout outings, the original
batteries lasted nearly a year. The one that really
died would actually burn brighter on the 'low' setting
than on 'high' at the very end. The internal
resistance of the battery had gone up enough that the
loss across it consumed enough power that the LED
could not achieve its maximum brightness. I don't
know how long the second set will last...they're out
of my 'change the battery in the smoke detector every
six months' box, but, hey, if I get six months' use
out of them, they're free...sort of...
Paul Wilson wrote:
For arctic expeditions most use a battery pack for the headlamp
that is worn inside the clothing so they work fine at low ambient
temps since the batteries do not get cold.
Alan Ritter wrote:
My experience with the NiMH batteries has been in moderate temps, down to
near freezing, but certainly not winter conditions. They seem to hold up
pretty well down to 32F.
Kevin Craig wrote:
NiMH's also suffer from the cold; to a lesser extent than
alkalines but MUCH more than lithiums. A matter of fractions
of ounces, but the Lithum batteries are also noticeably
lighter than either alkalines or NiMH's.
Paul Wilson wrote:
I just looked up alkaline capacities and the Eveready's best
alkaline is rated at 3135mAh and their new Lithium Photo is
listed at 2900mAh. This validates Monty's post. Who should
we believe, Eveready or various testimonials?
Consumers Reports tested alkaline's and said that the
Duracell Ultra is significantly better than the Eveready.
The cost at Walmart is 0.95 vs 2.48 each,less for the
alkalines when on sale. Lithiums seem to be pretty hard
to find in the out of the way places I tend to go.
Not many Walmarts or camera shops.
Another observation:
The Lithiums at 2900mAh and weigh 0.51 oz, Eveready alkaline
3135mAh, 0.81 oz and Duracell Ultra, 0.95 oz (all per battery).
Or 7647mAh/Oz, and 3870mAh/Oz. A huge difference.
Don't confuse [lithium batteries in] this discussion with the
[rechargeable] lithium-ion batteries that come in many electronic
gadgets these days. They are not the same as the Eveready Photo lithium's.
Alan Ritter wrote:
When you buy NiMH batteries, be careful of capacities (printed on the battery).
The older Energizer AA NiMHs were only 1200 mAH and my Nikon 990 eats them awfully
fast. Radio Shack has 1600 mAH AA cells. The latest Energizers (blue and gold
color scheme) are 1600 mAH, but I haven't tried them yet.
Thomas has some 1700 mAH AA cells, but they're pricey...
Ed Lulofs wrote:
On a per weight basis, [lithium batteries] have 4 times as much
energy per gram [as standard alkalines].
So I only use lithium batteries when backpacking.
I gave up on my Tikka because AAAs are not available in lithium.
I think that lithium [batteries] and LEDs are the most weight efficient
and reliable light source.
Craig Smith wrote:
The capacity of NiMH batteries has been slowly increasing. The best ones you
can buy in most stores now are 1500 or 1600 mA hours. But if you go to the
internet or some camera stores, look for batteries branded as either MaHa or
Quest. These are available in 1700 and 1800 mA hours for AA size. Quest also
makes a very nice charger for them that will charge 2 or 4 AAs or AAAs with
either AC or car battery. I highly recommend these products - have used them
extensively with both GPS and digital photo gear.
Monty Smith wrote:
In prepping for Denali, I wanted to find out how different batteries
performed in the cold, both for storage as well as power output. I found
much good info at Eveready's website, and the following is a summary of the
tables and graphs.
Summary:
Richard Booth wrote:
I have shifted to NiMH for most applications in order to cut down on the
disposing of batteries. They work well in cameras, headlamps, gps, etc.
Just a personal preference.
Alkaline:
Paul Wilson wrote:
We all use AA batteries for our gadgets such as head lamps, GPS
receivers, radios and the like. The typical alkaline batteries
have been the most popular since they are readily available and
can be found discounted at many big box stores. Recently, Eveready
came out with new chemistry, 'Lithium/iron Disulfide (Li/FeS2)'.
The proper name for this battery is 'Eveready Photo Lithium'.
This new battery comes from the store with an initial voltage of
1.775 volts average compared to the Duracell Ultra of 1.61 volts average.
This portends that the lithium battery will perform better due to higher potential.
Lithium batteries are also well known for their low temperature and
long shelf life as well as much higher cost.
In the sport plane field the subject came up as pilots often use the
same stuff climbers use. So, one of the electrical experts decided to
test a bunch of batteries. I provided him with the extra batteries so
low temperature data could be collected. This person Is Bob Nuckolls III.
What he did was write software for his laptop and wire up the batteries
to allow them to be discharged through a 5 ohm load each to a level of
1.0 and 0.8 volts at room temperature and in his freezer. Let's examine
the numerical data in table 1 which resulted from the testing.
Standard alkaline battery comparisons at 20 C:
Lithium battery comparisons at 20 C:
Comparisons at -20 C:
Conclusions:
Click here for a
larger bitmap, or
scalable PDF version
of this table.
The real numbers from the computer are as follows:
The lithium took 30,500 seconds to drop to 0.9 volts at -20 C and it ran 33,680 seconds at room temperature.
The Duracell Ultra poops out at 8,800 seconds at -20 C and it runs 31,430 seconds at room temperature.
Notes:
carrying spares is a function of battery size/weight/type
GPS (etc) battery meter shows remaining life incorrectly for NiMH
longest-lasting battery is different in high vs. low current applications
longest-lasting battery is different in moderate vs. low temperature applications
even in ideal conditions, capacity varies with battery type
Battery Life (summarized from battery technical info taken from Eveready's website)
General notes on all batteries:
Batteries described here in detail are all non-rechargeable 1.5V AA
batteries, since that's what commonly used in our equipment, although
there's a quick mention of rechargeables at the end. Storage for all
batteries is down to -40F, but operating range varies widely, as does
performance at various temperatures - only cold temperature info is
provided. Although % storage capacity loss per year (shelf life) varies
widely by both temperature and battery type, if being stored at 0C the worst
battery (carbon-zinc) only loses 3% per year, so cold storage while on a
climb won't affect any battery's capacity, but will affect its power
output. Internal resistance, or the ability to maintain constant voltage as
the power demand increases, is generally low (constant voltage) for all
types except carbon-zinc. Power output varies from 950mAh to over 3000mAh.
Definitions:
Carbon-zinc
These are the plain, old-fashioned batteries, and have power of 950mAh with
a sloping discharge curve. Operating range is only down to 20degF and shelf
life is 1/3 to 1/10th of other types - about 3.5 years. They perform very
poorly at low temperature; at -5degF their service life is decreased by 75%
and output drops quickly as the temperature drops below room temperature.
On the plus side, they're cheap.
Alkaline
The Energizer has 2850mAh and the new Energizer e2 has about 15% more, or
3135mAh, and both have a sloping discharge curve. Operating range is down to
0degF and shelf life is 10+ years. They're not much better at low
temperature; at -5degF their service life is decreased by 60% and output
drops quickly as the temperature drops below room temperature. Although
they're more expensive, they're comparable to carbon-zinc in terms of cost
per hour of use.
Silver oxide
These are the small (dime-shaped) batteries often used in cameras and
calculators. Since they don't come in AA size, comparative power output is
meaningless, but they have a flat discharge curve. Operating range is down
to 14degF and shelf life is 10+ years. They're a better at low temperature;
at -5degF their service life is decreased by 50%. Output decreases slowly
until about 40degF but below that output drops quickly.
Lithium
Lithium AAs won't outlast alkaline, with equivalent power of 2900mAh but
with a flat discharge curve. Operating range is down to -40degF and shelf
life is 10+ years. They're significantly better at low temperature; at
-5degF their service life only is decreased by 20% and output decreases
slowly as the temperature drops. They're also much more expensive.
Rechargable batteries
NiCads and Nickel-metal-hydrides have less storage capacity - only half that
of alkalines, they supply lower voltage, and also drain much faster by
themselves. They do not perform well at low temperatures, roughly
equivalent to carbon-zinc.
Ok. Here is my $.02 on batteries.
Primary cell (not rechargeable). These have good shelf life, that
is they can be left around for years and still maintain good capacity. Poor
high current drain applications such as digital cameras but ok for
headlamps. Poor low temperature performance. Checking the capacity
specifications should reveal the rate, in a fraction of the capacity, that
they can be discharged. Usually .1C or for a 2500 mahour battery this would
be 250 ma for about ten hours at room temperature. Typical capacities are
2500 mahour.
NiMH:
Nickle metal hydride. Secondary cell (re-chargeable). These have
poor shelf life and will supposedly discharge in some months. I use these
in a digtal camera and they work well for months in this application going
through hundreds of pictures. A set of alakalines will barely make it to
about 40 pictures. NiMH are initially expensive but if re-charged about ten
times the cost is approximately the same as alkaline. The weight of the
NiMH batteries is about the same as alakaline. Typical high performance
cells run about 1700 mahour which is less than alkaline. My NiMH batteries
in my digital camera worked ok at -17F winter ice climbing. NiMH is
supposedly better than alkaline at cold temperatures but the curves only
modestly support this. One observation: get NiMH from a reputable source.
They are sold by all sorts of bogus types. Try Thomas Distributing for Maha
batteries and charger.
Lithium:
The variations of lithium batteries is huge and the teminal
voltages come in a wide selection. The equivalent to the 1.5 V alakaline
seems to be made only by Eveready. These are primary cells and cannot be
re-charged. The cost is large, about $2.50 per battery, but they weigh
about 60% of an alkaline and have about 3x the capacity. They work well in
cameras. They are supposedly the low temperature kings. They have
tremendous shelf life. I used a pair of surplus lithium batteries, bought
for $.50, to run a piece of ham gear. They were at least ten years old and
provided about 90% of the capacity after years of being stored in a hot
garage.
AA Battery Endurance Testing
The Duracell Ultra has the greatest energy at 2.42 watt-hours,
but the Dollar General batteries have the lowest cost, $0.12 per battery.
So the discount house gives good value but not as long a life compared to
the best alkaline battery which is the Duracell Ultra.
The lithium battery compared to the Duracell Ultra has 31% more energy and is 120.% $/watt-hour higher in cost.
The lithium battery compared to the Dollar General has 52% more energy and is 558% $/watt-hour higher in cost.
The Lithium has 272% more energy than the Duracell and costs 23% $/watt-hour LOWER in cost.
If the temperature is real cold, use lithium instead of Duracell Ultra for lower cost and for longer life.
At normal temperatures and long trips select Duracell Ultra.
For shorter trips select the cheapest alkaline batteries you can get and change them as required.

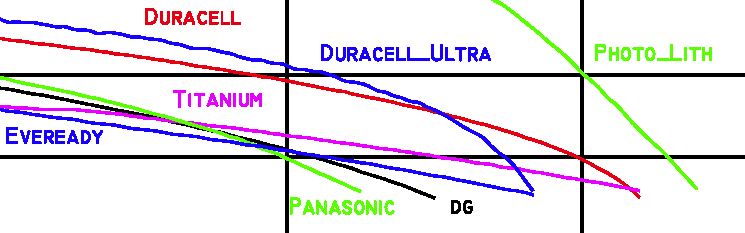
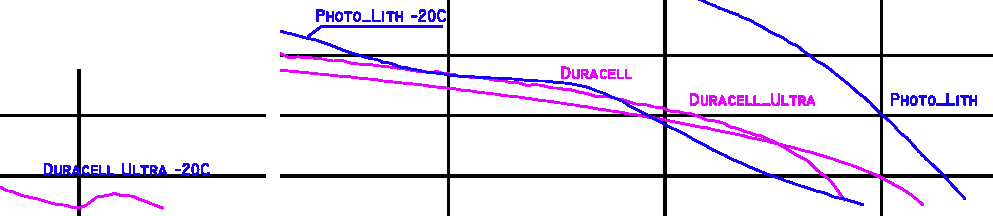
Battery Life, at Cold Temperatures - overall graph
Click here for a
larger bitmap, or
scalable PDF version
of this graph.
Part of the graph above, zoomed in to read brand names:
Click here for a
larger bitmap, or
scalable PDF version
of this graph.
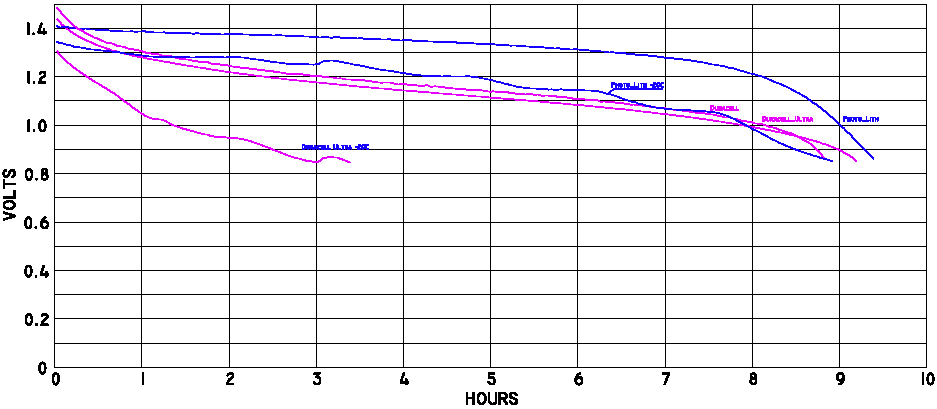
Battery Life, at Warm Temperatures
Click here for a
larger bitmap, or
scalable PDF version
of this graph.
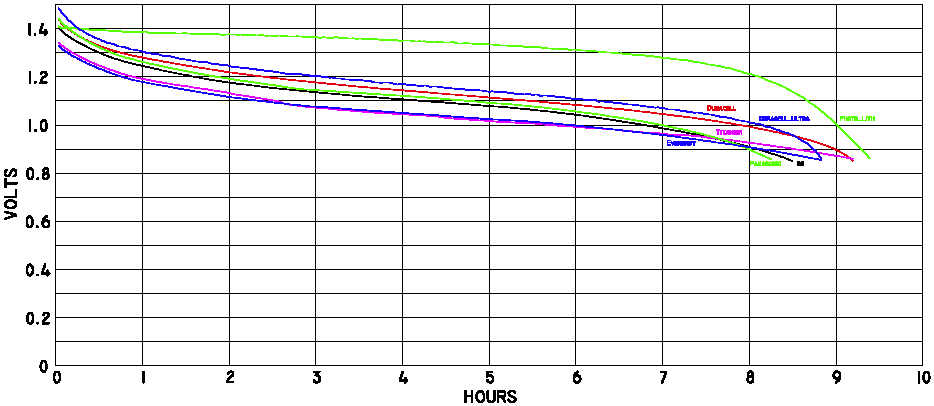
Part of the graph above, zoomed in to read brand names